GARDEN ROUTE | KAROO NEWS - A "healthy pipeline" of therapies for the treatment of Covid-19 is either under review or has already been approved by the South African Health Products Regulatory Authority (Sahpra), according to CEO Dr Boitumelo Semete.
She spoke during a webinar on Monday 21 February held to provide an update on therapies available.
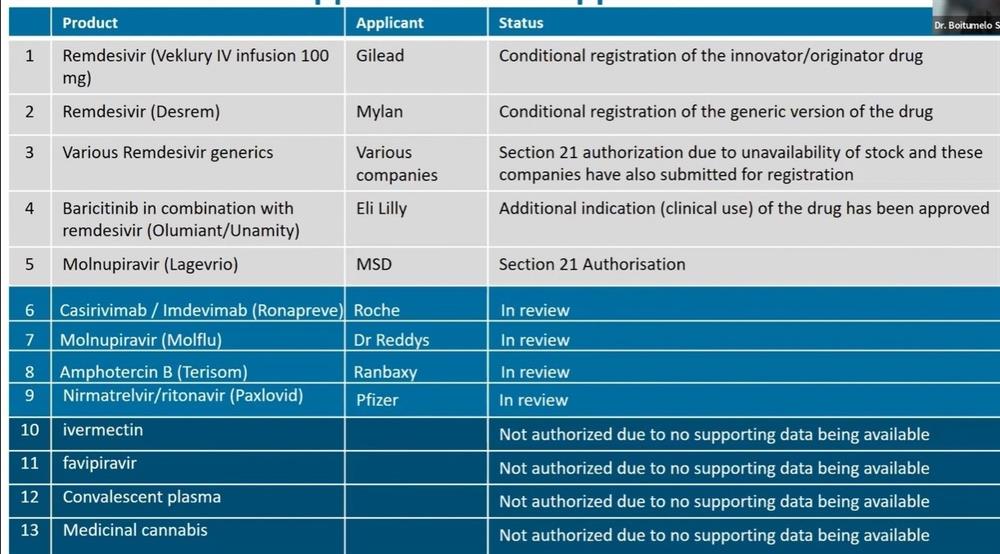
Semete said a number of broad-acting existing drugs are increasingly being repurposed to treat Covid-19.
Remdesivir from the company Gilead and a generic version of the drug called Desrem (from Mylan) have received conditional registration.
Various other Remdesivir generics, of which there is limited stock available, have been granted Section 21 authorisation (for limited use on a limited number of patients). The companies applying in the latter case have at the same time applied for registration.
Baricitinib was also registered for use in combination with Remdisivir.
Section 21 authorisation was granted to Molnupiravir (original version from MSD) and a generic version from the company Dr Reddys is currently under review.
Molnupiravir is however not recommended by the National Department of Health as a Covid-19 therapy and the department will not acquire the drug for public sector use, according to Prof Jeremy Nel, head of the infectious diseases division at Wits University and a member of the ministerial advisory committee (MAC) on Covid-19.
Nel said one of the reasons is that the drug has to be given within the first five days of developing symptoms. To get tested to confirm infection and then obtain the medication, all within five days, can be challenging.
The drug is furthermore prescribed only for high-risk individuals and is therefore not for the general population who have low levels of complications.
It would be a waste for the department to make the drug accessible to people who may not need it.
Semete said Casirivimab, a monoclonal antibody drug from Roche, is currently under review. Ivermectin and medicinal cannabis have not been authorised because sufficient supporting data is not available.
Semete said in addition to reviewing data from applicant companies, Sahpra also takes into consideration the assessments of other regulators such as the Food and Drug Administration (FDA) and their emergency-use status at the World Health Organisation (WHO).
Registered vaccines
Vaccines that to date have received full registration with conditions are Pfizer, Johnson & Johnson and the Chinese vaccines Sinopharm and MC Pharma.
The conditional use implies that continuous monitoring and implementation of a risk management plan as well as continuous reporting to Sahpra must be adhered to. The efficacy of the vaccines against any emerging variant of Covid-19 must also be monitored, said Semete.
Vaccination intervals shortened, heterologous doses allowed
The national health department announced in a circular on Monday 21 February that the interval between the first and second doses of the Pfizer vaccine has been reduced from 42 days to 21 days.
 Medicinal cannabis and ivermectin have not been approved as Covid therapies because of a lack of supporting data, according to Sahpra.
Medicinal cannabis and ivermectin have not been approved as Covid therapies because of a lack of supporting data, according to Sahpra.
Furthermore, the 180-day interval between the second dose and the booster dose was halved to 90 days.
The heterologous use of booster doses was also approved and individuals who have received two Pfizer doses may now receive a booster dose of the Johnson & Johnson (J&J) vaccine 90 days after the second Pfizer dose, and those who have received one dose of the J&J may receive a booster dose of the same vaccine or of Pfizer after an interval of 60 days.
Deciding which vaccine to administer as a booster should be guided by vaccine availability, said the department. If both vaccines are available, homologous boosting should be preferred, unless the person being vaccinated requests to receive a heterologous booster dose, or has experienced an adverse event after immunisation.
482 active cases in Garden Route
The number of reported active cases in the Garden Route is still declining. As at Wednesday 23 February, there were 482 cases (George 198, Mossel Bay 81, Oudtshoorn 48, Hessequa 75, Knysna 39, Bitou 38 and Kannaland 3) compared with 545 as at Tuesday 15 February.
 Sahpra CEO Dr Boitumelo Semete during the webinar on Covid-19 therapies.
Sahpra CEO Dr Boitumelo Semete during the webinar on Covid-19 therapies.
'We bring you the latest Garden Route, Hessequa, Karoo news'
















